NEWS
BioForward’s leading voices in the industry sharing their knowledge with news outlets and us, the biohealth community.
BioForward Wisconsin closely follows news stories about our members and invites them to contribute blogs and profiles to inform and advance the Wisconsin biohealth community. View the most recent entries below and use the search bar at the bottom of the page to look up a specific company or keyword.
Have some relevant and timely news or a press release you want to see on the BioForward website? Fill out this form to submit it!
We are looking for member-company knowledge leaders who are willing to represent their companies and the Wisconsin biohealth industry in the media. Let us know if you are willing to be added to the list.
The Wisconsin biohealth industry needs a hero! We are looking for well-known/famous spokespersons that your organization may have a relationship with who may be able to represent Wisconsin and the biohealth industry. Submit a suggestion.
BioForward & Members in the News

BioForward Wisconsin Releases 2023 Annual Report Highlighting Major Accomplishments
FOR IMMEDIATE RELEASE MADISON,...
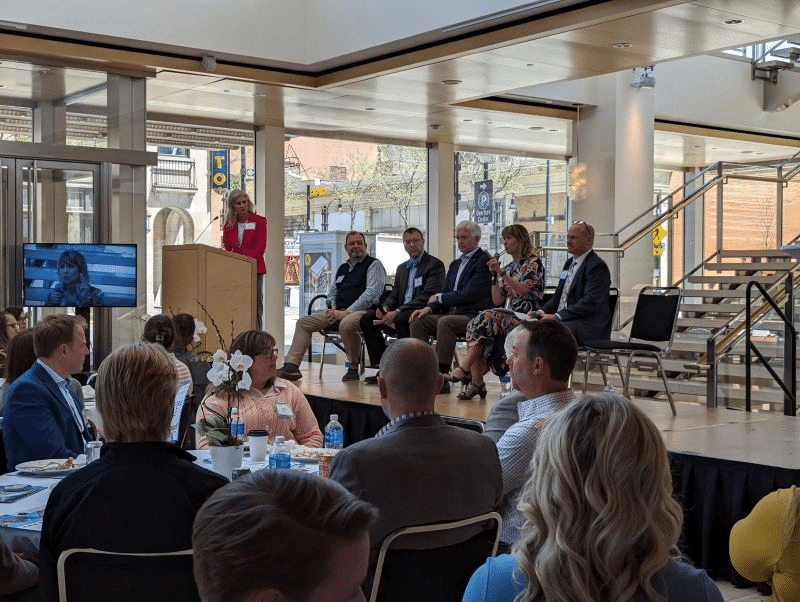
BioForward Unveiling the Future of Wisconsin Biohealth: Wisconsin Biohealth Tech Hub Projects Set to Transform Personalized Medicine
FOR IMMEDIATE RELEASE — May 7, 2023 MADISON, Wis — BioForward, the leading advocate for Wisconsin's thriving biohealth sector, hosted its...

BioForward Presents 2024 Community Service Awards: Honoring Excellence in Philanthropy and Impact
FOR IMMEDIATE RELEASE- May 6, 2024 MADISON, Wis — BioForward, the leading advocate for Wisconsin's thriving biohealth sector, proudly unveiled...
Microbial Discovery Group expands into third facility
A growing Milwaukee-area company that develops products in the microbial solutions market has expanded into its third facility. Oak...
BioForward Blog & Member Profiles & Blogs
Would your organization like to submit a profile or blog for inclusion on the BioForward website? Please fill out this form for a profile or this one for a blog post.
Wisconsin’s Manufacturing Strength Boosts Biohealth Sector
Wisconsin’s strength in biohealth extends well beyond the laboratories where researchers are working to develop new drugs and therapies. The...
BioForward’s End of Year Celebration: A Night of Innovation, Recognition, and Cheer
The Wisconsin biohealth industry culminated the year in style at BioForward’s End of Year Celebration on December 12th, held at the...

Wisconsin Biohealth Summit: The Cutting-Edge World of Personalized Medicine
The 2023 Wisconsin Biohealth Summit concluded with a remarkable closing keynote session that took attendees on a captivating journey through...

Wisconsin Biohealth Summit: Emerging Growth Companies Unveil the Future of Personalized Medicine
The 2023 Wisconsin Biohealth Summit embarked on an exciting journey into the realm of personalized medicine in Wisconsin during the second...
